
When we think of matter, our minds often conjure images of solid objects, liquid substances, and perhaps even ethereal concepts like plasma. Yet, there’s a fascinating state of matter that often escapes our immediate attention — the gaseous state. In this exploration, we’ll delve into the mysterious world of gases, uncovering their properties, behaviours, and the intriguing laws that govern them. Join us on this educational journey, enriched by the insights of the best chemistry tuition in Singapore, where the invisible realm of gases comes to life.
What is Gas?
Before we venture into the complexities of the gaseous state, let’s start with the basics: what exactly is gas? In scientific terms, gas is one of the three fundamental states of matter, alongside solids and liquids. Unlike solids, which maintain a fixed shape and volume, and liquids, which have a definite volume but take the shape of their container, gases possess neither a fixed shape nor volume. Instead, they expand to fill the space available to them.
A Brief of Gaseous State:
The gaseous state is a state of matter characterized by particles that are widely separated and move freely at high speeds. This inherent freedom of movement allows gases to diffuse and mix rapidly, making them essential players in various natural and industrial processes. From the oxygen we breathe to the carbon dioxide we exhale, gases are omnipresent, shaping our world in ways both seen and unseen.
Properties of Gaseous States:
Gases, the elusive and dynamic state of matter, exhibit a set of distinctive properties that define their behavior and interactions. In the gaseous state, particles are characterized by their wide separation and high-speed, random motion. These unique characteristics contribute to the versatility of gases, impacting various natural and industrial processes.
Now, let’s delve into the specific properties that make gases a fascinating subject of study:
- Compressibility: Gases can be compressed into smaller volumes under pressure and expand when pressure is released.
- Temperature Responsiveness: Gases are highly responsive to changes in temperature, as described by Charles’ Law, where the volume is directly proportional to temperature at constant pressure.
- Diffusion: Gases exhibit spontaneous spreading and mixing with other gases, a fundamental property essential in biological and chemical phenomena.
Kinetic Molecular Theory:
As we plunge deeper into the realm of gases, the Kinetic Molecular Theory (KMT) becomes our guiding light, shedding insight into the microscopic dance of gas particles. According to KMT, gases are composed of particles in constant, random motion, resembling a lively ballet on the molecular scale. The theory posits that the pressure exerted by a gas is a result of these particles colliding with the walls of their container.
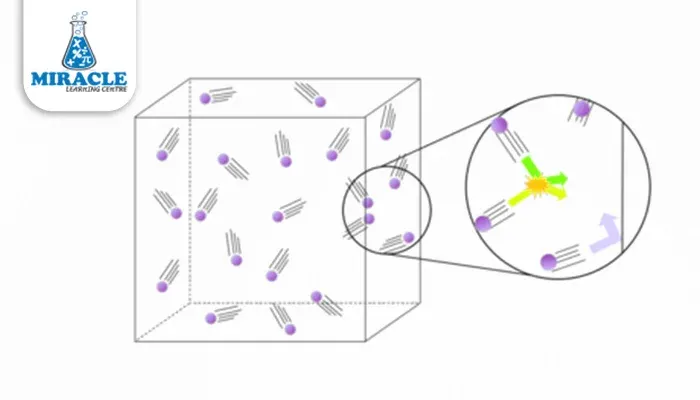
In a more concrete sense, the kinetic energy (KE) of these gas particles can be mathematically expressed through the formula:
KE =1/2 mv2
Here,
m represents the mass of the gas particle, and v denotes its velocity. This equation encapsulates the essence of KMT, illustrating that the average kinetic energy of gas particles is directly proportional to the temperature of the gas. In simpler terms, as the temperature rises, so does the average kinetic energy of the gas particles, intensifying their frenetic motion and consequently elevating the pressure exerted on the container walls.
How does our chemistry tuition provide you with detailed knowledge about it?
Embarking on a journey to comprehend the gaseous state might seem daunting, but fear not — the right guidance can illuminate this path. Our chemistry tuition is designed not only to impart theoretical knowledge but also to provide hands-on experiences that solidify your understanding.
Through interactive sessions, practical experiments, and engaging discussions, our chemistry tutors break down complex concepts into digestible nuggets of wisdom. Whether it’s grasping the intricacies of gas laws or visualizing the dynamic nature of gas particles through simulations, our chemistry tuition ensures that you not only learn about the gaseous state but develop a profound understanding that extends beyond the confines of textbooks.
Moreover, our commitment to fostering curiosity and critical thinking, coupled with the expertise of the best chemistry tuition in Singapore, means you’ll not only memorize facts but actively explore the ‘whys’ and ‘hows’ that make the gaseous state a captivating subject. By demystifying the seemingly abstract principles governing gases, our tuition transforms the learning process into an exciting adventure, where every revelation sparks a newfound appreciation for the invisible world of gases.
Conclusion:
In conclusion, the gaseous state, though often overlooked, is a realm of matter filled with intrigue and complexity. From the relentless motion of gas particles to the elegant laws that govern their behaviour, understanding gases opens up a world of possibilities and applications. With the right guidance, such as our chemistry tuition provides, the journey into the gaseous state becomes not only comprehensible but genuinely captivating. So, let’s embrace the invisible and embark on a quest to unravel the secrets of the gaseous state, where the mundane transforms into the extraordinary.
